


Maintaining sterility in ATMP manufacturing is vital, but traditional tests are too slow (up to 14 days) for these short shelf-life products.
Red One™ accelerates detection by staining microorganisms based on their metabolic activity.
To distinguish them from active mammalian cells, Redberry uses a reagent that selectively lyses cells while preserving microorganisms, allowing rapid and accurate differentiation based on automated Solid-Phase Cytometry (SPC).
Maintaining sterility in ATMP manufacturing is vital, but traditional tests are too slow (up to 14 days) for these short shelf-life products.
Red One™ accelerates detection by staining microorganisms based on their metabolic activity.
To distinguish them from active mammalian cells, Redberry uses a reagent that selectively lyses cells while preserving microorganisms, allowing rapid and accurate differentiation based on automated Solid-Phase Cytometry (SPC).



The selective lysis step can be directly performed on fresh culture of cells.
This technique’s efficacy was validated on various cell types, including HeLa, Jurkat, Stem, CAR-T, and CHO cells.
In this case, the DVC application provides a direct count (excluding spores) in 10 minutes on samples ranging from 500µL to 2mL.
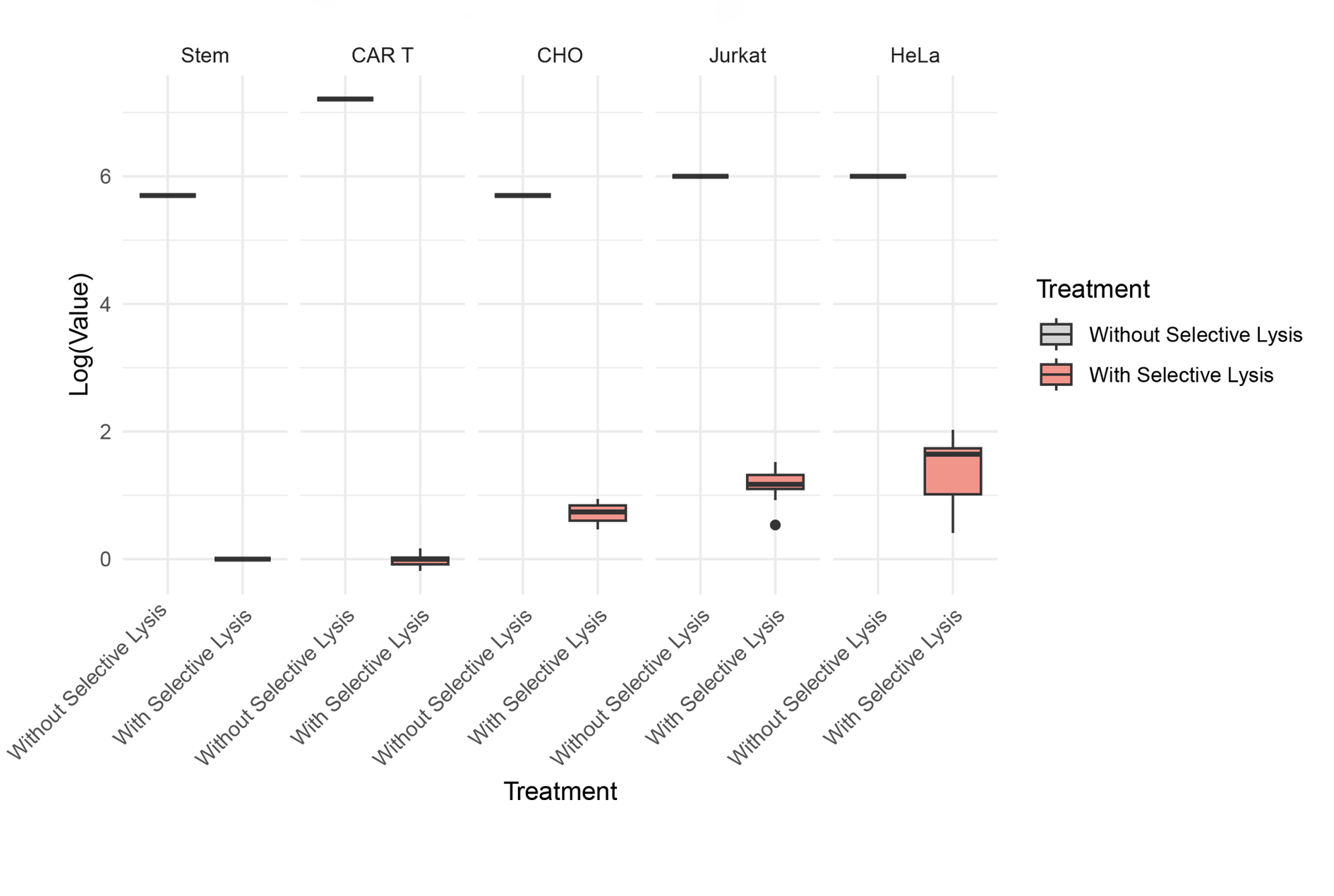
Efficiency of lysis on mammalian cells
Detection reduction >99,99%
The selective lysis step can be directly performed on fresh culture of cells.
This technique’s efficacy was validated on various cell types, including HeLa, Jurkat, Stem, CAR-T, and CHO cells.
In this case, the DVC application provides a direct count (excluding spores) in 10 minutes on samples ranging from 500µL to 2mL.


Efficiency of lysis on mammalian cells
Detection reduction >99,99%
The selective lysis step can be directly performed on fresh culture of cells.
This technique’s efficacy was validated on various cell types, including HeLa, Jurkat, Stem, CAR-T, and CHO cells.
In this case, the DVC application provides a direct count (excluding spores) in 10 minutes on samples ranging from 500µL to 2mL.


Efficiency of lysis on mammalian cells
Detection reduction >99,99%



It complies with the European Pharmacopoeia 2.6.27, considering a detection limit of 100 CFU.
The process requires an activation phase similar to the Bioburden application.
Anaerobic strains are detected after 6 hours.

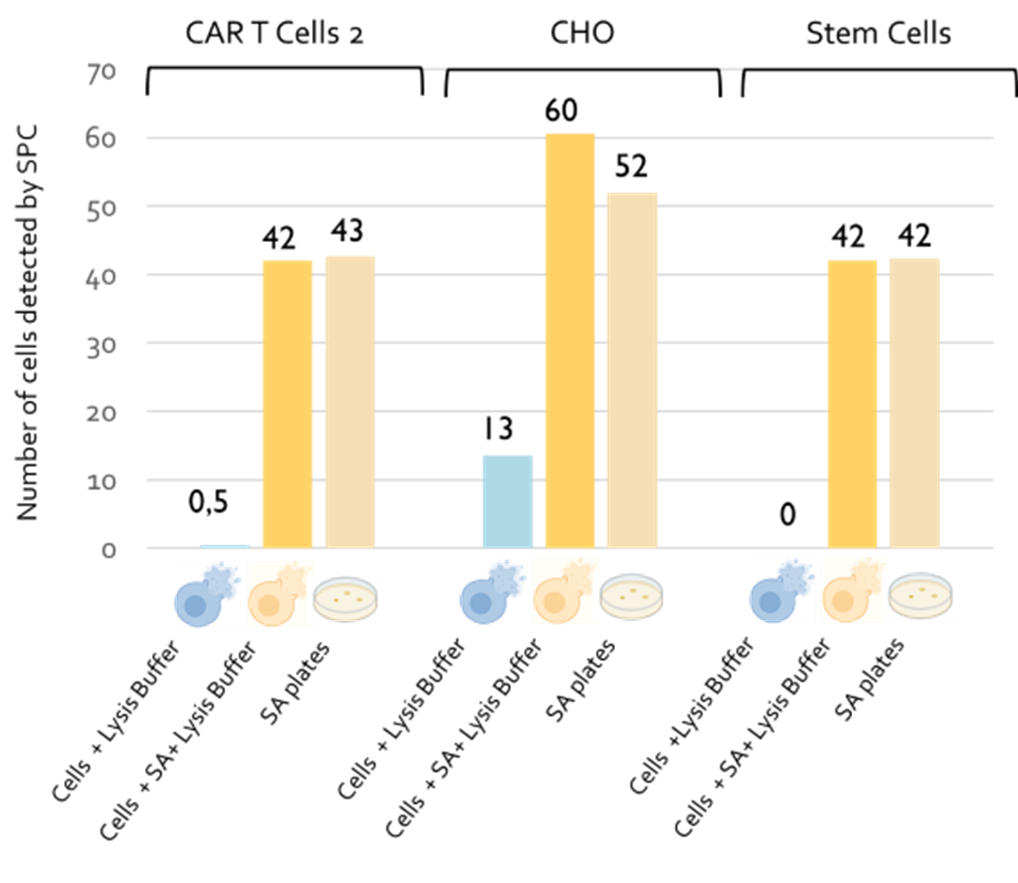
Detection of 50 S. aureus (SA) spiked in cellular products 50 CFU of S. aureus spiked in cellular products after lysis treatment
It complies with the European Pharmacopoeia 2.6.27, considering a detection limit of 100 CFU.
The process requires an activation phase similar to the Bioburden application.
Anaerobic strains are detected after 6 hours.


Detection of 50 S. aureus (SA) spiked in cellular products 50 CFU of S. aureus spiked in cellular products after lysis treatment
It complies with the European Pharmacopoeia 2.6.27, considering a detection limit of 100 CFU.
The process requires an activation phase similar to the Bioburden application.
Anaerobic strains are detected after 6 hours.


Detection of 50 S. aureus (SA) spiked in cellular products 50 CFU of S. aureus spiked in cellular products after lysis treatment



The workflow strictly relies on Red One™ Sterility Sample & Analysis application based on the compendial sample preparation in FTM and TSB media.
After the selective lysis step, a small sample (1 to 10mL) is analyzed on Red One™, with results delivered in 96 hours (instead of 14 days).
Further identification remains possible with the content remaining in bottles.



