Red One™ Pharmaceuticals Standard Products
Red One™ Pharmaceuticals Standard Products


The Red One™ Direct Viable Count application provides a direct analysis within 10 minutes for the total viable cell count, for samples ranging from 10µL to 250mL.
Simply drop the sample on the cap, and it’s done! Its unprecedented level of sensitivity,detecting down to 10 cells per sample, surpasses the capabilities of flow cytometry and ATP tests (with a typical limit of detection of 1,000 cells/mL).
This application has demonstrated consistency with traditional 5-day counts on R2A agar plates for water loops.
Other applications include seed lot enumeration (ex: mycobacteria), in-process or environmental testing, and more.
The Red One™ Direct Viable Count application provides a direct analysis within 10 minutes for the total viable cell count, for samples ranging from 10 µL to 250 mL.
Simply drop the sample on the cap, and it’s done! Its unprecedented level of sensitivity,detecting down to 10 cells per sample, surpasses the capabilities of flow cytometry and ATP tests (with a typical limit of detection of 1,000 cells/mL).
This application has demonstrated consistency with traditional 5-day counts on R2A plates for water loops.
Other applications include lot enumeration (ex: mycobacteria), in-process or environmental testing, and more.
The Red One™ Direct Viable Count application provides a direct analysis within 10 minutes for the total viable cell count, for samples ranging from 10µL to 250mL.
Simply drop the sample on the cap, and it’s done! Its unprecedented level of sensitivity,detecting down to 10 cells per sample, surpasses the capabilities of flow cytometry and ATP tests (with a typical limit of detection of 1,000 cells/mL).
This application has demonstrated consistency with traditional 5-day counts on R2A agar plates for water loops.
Other applications include seed lot enumeration (ex: mycobacteria), in-process or environmental testing, and more.

The Red One™ Direct Viable Count application provides a direct analysis within 10 minutes for the total viable cell count, for samples ranging from 10µL to 250mL.
Simply drop the sample on the cap, and it’s done! Its unprecedented level of sensitivity,detecting down to 10 cells per sample, surpasses the capabilities of flow cytometry and ATP tests (with a typical limit of detection of 1,000 cells/mL).
This application has demonstrated consistency with traditional 5-day counts on R2A agar plates for water loops.
Other applications include seed lot enumeration (ex: mycobacteria), in-process or environmental testing, and more.
The Red One™ Direct Viable Count application provides a direct analysis within 10 minutes for the total viable cell count, for samples ranging from 10 µL to 250 mL.
Simply drop the sample on the cap, and it’s done! Its unprecedented level of sensitivity,detecting down to 10 cells per sample, surpasses the capabilities of flow cytometry and ATP tests (with a typical limit of detection of 1,000 cells/mL).
This application has demonstrated consistency with traditional 5-day counts on R2A plates for water loops.
Other applications include lot enumeration (ex: mycobacteria), in-process or environmental testing, and more.




The Red One™ Bioburden application is designed for the quantification of total aerobic mesophilic viable flora, including fungi and spores.
After the initial sample filtration onto the cap, an activation medium is added, followed by a 4 hours activation step to restore the metabolic activity of the microorganims, making it compliant with staining and detection.
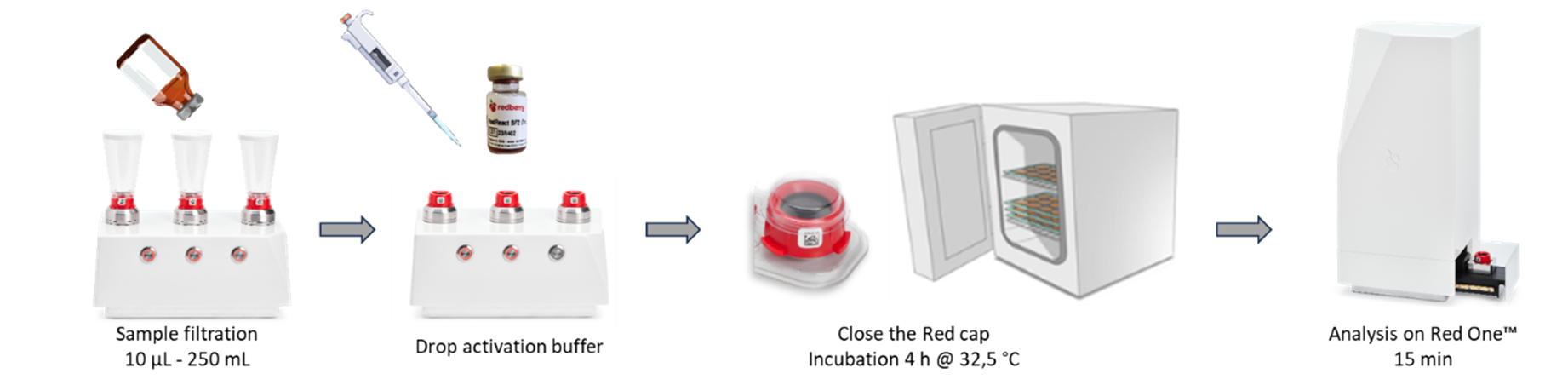
If a detection limit of 1 CFU and a post-analysis identification are required, the enrichment in the cap can be extended to 24 hours to obtain a qualitative result.
The Red One™ Bioburden application is designed for the quantification of total aerobic mesophilic viable flora, including fungi and spores.
After the initial sample filtration onto the cap, an activation medium is added, followed by a 4 hours activation step to restore the metabolic activity of the microorganims, making it compliant with staining and detection.

If a detection limit of 1 CFU and a post-analysis identification are required, the enrichment in the cap can be extended to 24 hours to obtain a qualitative result.



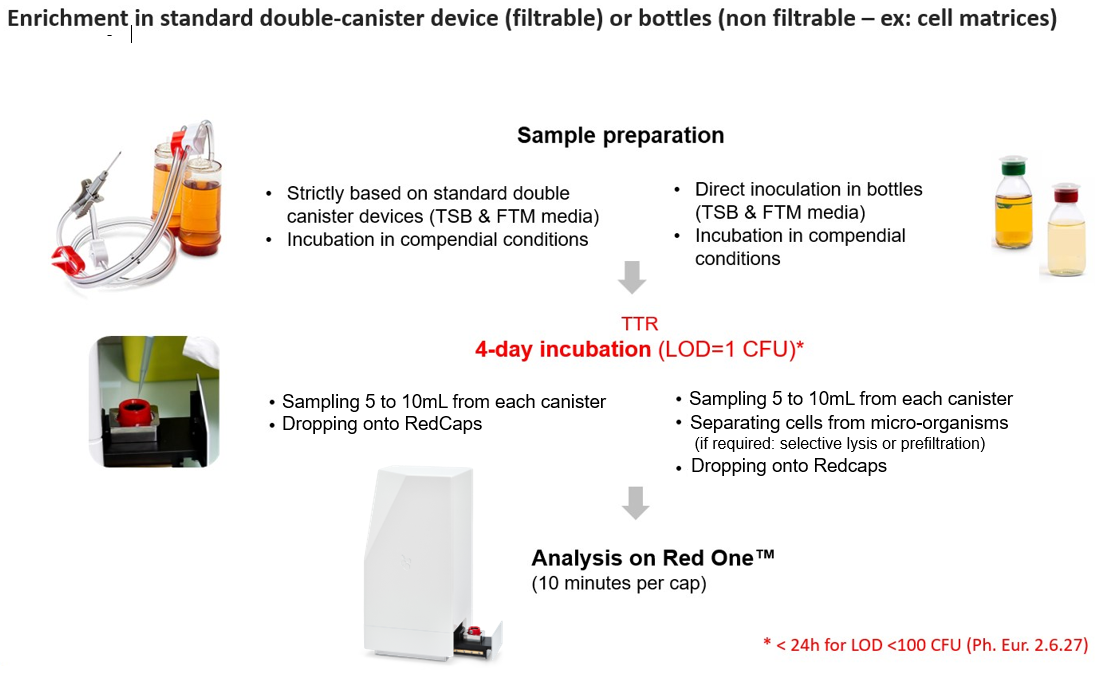
The Red One™ Sterility Sample & Analysis application strictly relies on compendial sample preparation in a standard double-canister device. After a 96-hour incubation period, a small volume from the canister, typically ranging from 1 to 10mL, is sampled and analyzed using Red One™.
The Time to Result (TTR) is 96 hours and Red One™ delivers a qualitative result (presence/absence). This short TTR is achieved through the system’s unique capability to detect single microbial cells. In comparison, traditional visual inspection methods require a significantly higher microbial population (typically on the order of 108 cells) for confirmation of a positive result.
Primary Validation Package is available (PE 5.1.6 and PDA TR.33) and Redberry offers support for Validation for Intended Use (PQ).


The Red One™ Sterility Sample & Analysis application strictly relies on compendial sample preparation in a standard double-canister device. After a 96-hour incubation period, a small volume from the canister, typically ranging from 1 to 10mL, is sampled and analyzed using Red One™.
The Time to Result (TTR) is 96 hours and Red One™ delivers a qualitative result (presence/absence). This short TTR is achieved through the system’s unique capability to detect single microbial cells. In comparison, traditional visual inspection methods require a significantly higher microbial population (typically on the order of 108 cells) for confirmation of a positive result.
Primary Validation Package is available (PE 5.1.6 and PDA TR.33) and Redberry offers support for Validation for Intended Use (PQ).
The Red One™ Sterility Sample & Analysis application strictly relies on compendial sample preparation in a standard double-canister device. After a 96-hour incubation period, a small volume from the canister, typically ranging from 1 to 10mL, is sampled and analyzed using Red One™.
The Time to Result (TTR) is 96 hours and Red One™ delivers a qualitative result (presence/absence). This short TTR is achieved through the system’s unique capability to detect single microbial cells. In comparison, traditional visual inspection methods require a significantly higher microbial population (typically on the order of 108 cells) for confirmation of a positive result.
Primary Validation Package is available (PE 5.1.6 and PDA TR.33) and Redberry offers support for Validation for Intended Use (PQ).


Available applications on Red One™ include rapid screening, bioburden and sterility testing. Time-To-Result (TTR) depends on the targeted LOD and the need of post-analysis identification.